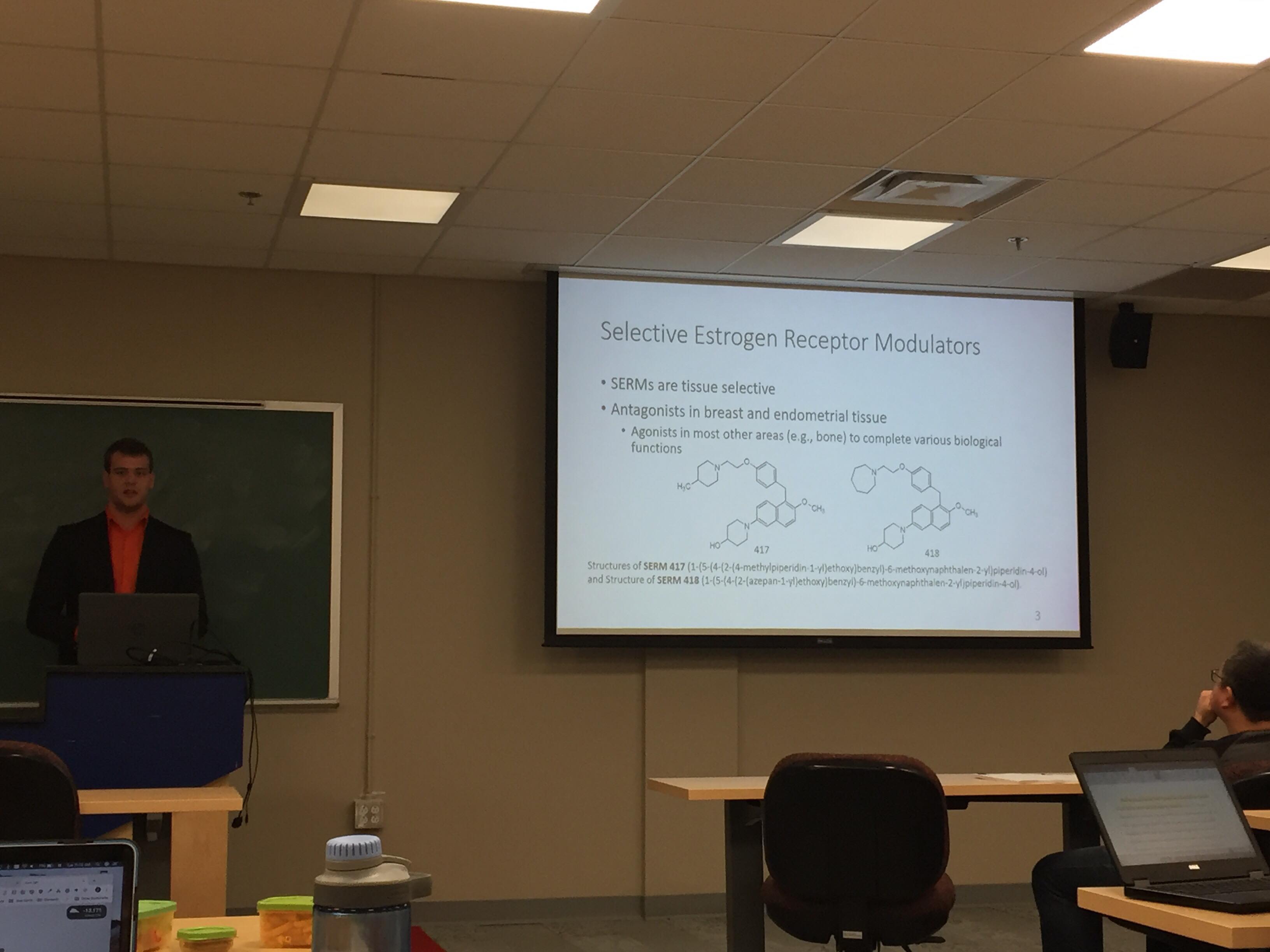
My name is Zachary Visser and I am a fourth-year student pursuing my Honours in chemistry and double major in biology. The nature of my degree has allowed me to complete molecular biology research under the supervision of Dr. Russell Easy as my honours project in chemistry. My project looks at the gene expression profiles of novel Selective Estrogen Receptor Modulators (SERMs for short) when administered in banded killifish (Fundulus diaphanous). The novel SERMs have been synthesized by Dr. Amitabh Jha (Acadia Chemistry) and his research team, showing promise in initial biological assays. The main research objective is to assess the mechanism of action of these drugs. Mechanism(s) of action could provide indication of efficacy and tissue selectivity as well as possible indications of deleterious side effects.
The novel SERMs are 6-(4-Hydroxypiperidino) naphthalen-2-ol-based, which essentially means they mimic the structure of the most common naturally occurring estrogen: 17β-estradiol. The need for SERMs is based on the conundrum of estrogen being essential for many biological functions, yet also being linked as a carcinogen (cancer-causing agent) for breast and uterine cancers. SERMs can be administered as an adjuvant hormone therapy in addition to other traditional cancer treatment options like surgery, radiation therapy, and chemotherapies. As the name suggests, SERMs (Selective Estrogen Receptor Modulators) are tissue selective, meaning they can cause varying effects in different parts of the body. Ideally, SERMs will cause agonistic (or estrogen-like) effects in many tissues of the body, carrying out those essential functions of estrogen. Yet, in breast and endometrial tissue, the SERMs should act as antagonists, inhibiting the growth and metastasis of cancerous tissue. There have been a few SERMs which have been used in clinical trials; however, the trials have been discontinued due to side effects including thrombosis, fatal strokes, and increased incidence of endometrial cancers. As a result, there is still the potential for SERMs which have the proper tissue selectivity and do not include deleterious side effects.
My work has included looking at gene pathways being modified through administration of the SERMs. I have accomplished this through administering the SERMS, collecting tissues and subsequently quantifying RNA. Since our genes are first transcribed into RNA (from our DNA) before they are translated into proteins, we can assess cellular function by quantifying RNA. Some of the genes of interest I have been looking at include cyclin D1, foxP3, and rap1gap. All these genes are considered oncogenes, meaning their mutations could lead to cancer. The genes mentioned previously have functions related to regulating the cell cycle (the growth and replication of cells), acting as a transcription factor (regulator of various gene pathways, known to be overexpressed in breast cancer), and signal transduction pathways (cell-cell communication) respectively. My work has shown that the novel SERMs have played a significant role in regulating cyclin D1 in gill tissue. The cell cycle gene was upregulated when the fish were administered both novel SERMs and 17β-estradiol (natural estrogen). This agonistic result was observed for gill tissue – an antagonistic (downregulation) response would be expected for the SERMs on other tissues (breast and endometrial).
Future work on this project would include looking at more tissues (including those where the SERMs should act as an antagonist) and more gene pathways. This would make the study completer and more robust, providing a more complete profile for the mechanism of action of these SERMs. This information would be useful for Dr. Jha and his research group for the movement of one of these novel SERMs to clinical trials, or potential future syntheses of more novel SERMs.
Zachary Visser is a fourth year (Honours) Chemistry and Biology student and member of the Men’s Varsity Soccer team